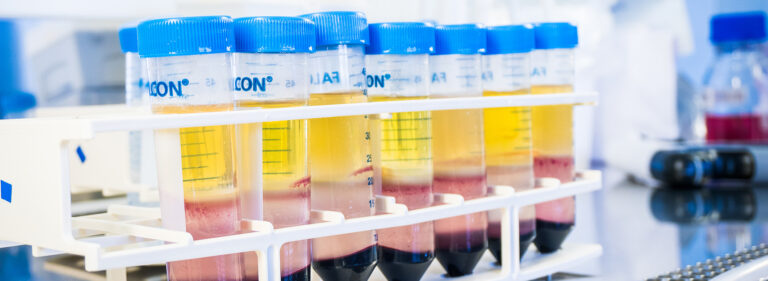
Biobank services for researchers
The Blood Service Biobank collects samples and data for research purposes from blood donors from all over Finland who have given their consent. Our collection of samples and related data can be released for high-quality scientific studies or approved by our organisation.

Our aim is to support and facilitate ethically acceptable high-quality scientific research within the Biobank’s research area by providing researchers a comprehensive collection of first-class material.
Our large base of donors, all of whom meet the blood donation criteria, together with the Blood Service’s strict quality requirements, serves to ensure the high quality of our sample collection.
What we offer for researchers
- A constantly continuously expanding collection
- High-quality samples and data from all areas of Finland
- Samples selected based on factors such as genotype, provided fresh or frozen
- Fresh samples as early as under 24 hours from donation
- Sample collection to meet research needs
- Longitudinal samples
- Expert services.
Our unique service
We provide fresh cells for research purposes, selected based on genetic variants or HLA type, from the whole of Finland within 24 hours!
You can find out more about this special service in Nature Communications.
Our specialists are here to help
The best way to reach us is to email us at biopankki@bloodservice.fi.
Kimmo Pitkänen
PhD, Biobank director. The Blood Service Biobank’s person in charge and head of unit.
Satu Koskela
PhD, Docent of Immunogenetics, biobank specialist. Processing of applications for material use, use of genomic data returned from studies.
Mirva Ikonen
MSc, Public health nurse. Processing of applications for material use, customer collaboration.