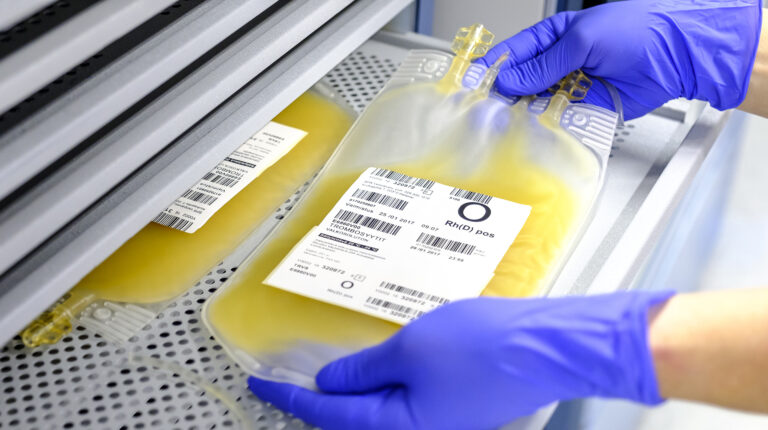
Bacterial Culture of Platelets
In spring 2025, the Finnish Red Cross Blood Service introduced bacterial culture testing for all platelet (thrombocyte) products. This procedure improves the microbial safety of the products and thereby enhances patient safety. As part of the reform, the shelf life of the products was extended from five to seven days.

Culture detects possible bacterial contamination
A sample is taken from each platelet product and cultured for bacteria before the product is released for hospital use. This process enables more effective identification of potential bacterial contamination and prevents contaminated products from being transfused to patients.
The need to introduce bacterial culture is based on improving the safety of blood products and patient care. It also ensures better availability of platelet products, reduces waste, and streamlines the logistics of blood products.

Extended shelf life improves inventory management
Platelet products are challenging to store due to their short shelf life. The extended use time increases flexibility in both blood product logistics and inventory management. For example, during holiday periods, platelet inventory management becomes easier.